Endothelial Dysfunction: The 'Silent Trigger' of Cardiovascular Disease
The root mechanism of endothelial dysfunction and our top tests to assess the risk factors for CVD.
Endothelial Dysfunction: The ‘Silent Trigger’ of Cardiovascular Disease
The root mechanism of endothelial dysfunction and our top 4 tests to assess the risk factors for CVD.
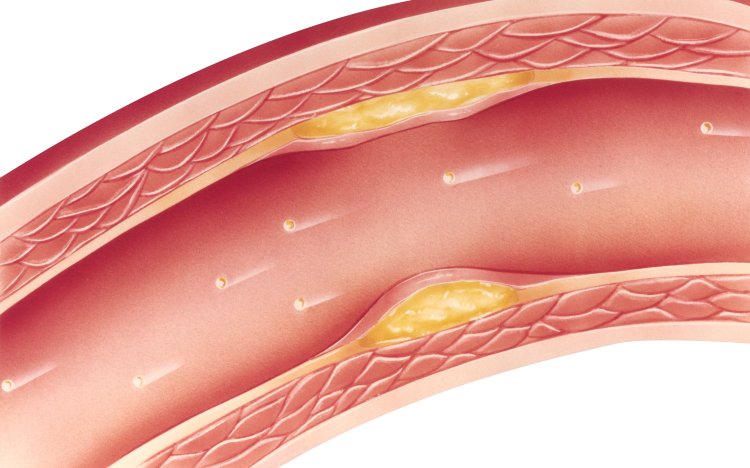
Cardiovascular disease (CVD) represents a huge burden to our healthcare system, costing the NHS an estimated 7.4 billion per year. It is the cause of 1 in 4 deaths in the UK, representing one death every 4 minutes. More than 80% of CVD-associated deaths are attributed to atherosclerosis, the excessive accumulation of lipids, cholesterol, inflammatory cells, and connective tissue in vessel walls (1). These plaques are often clinically silent, and that silence can be deadly.
Plaques and Atherosclerosis: What Are They?
The term atherosclerosis reflects the two principal components of a lesion; that is, ‘athero’ from the Greek word for gruel, which describes the necrotic core area at the base of the atherosclerotic plaque, and ‘sclerosis’ from the Greek word for hardening or induration, which describes the fibrotic cap at the luminal edge of the plaque.
Atherosclerotic lesions typically contain three components - cholesterol, in the form of cholesterol esters; cells, consisting mainly of smooth muscle cells and macrophages; and connective tissue composed of collagen, elastin and glycosaminoglycans.
Although cardiovascular mortality rates have almost halved in recent decades, in the area of atherosclerosis improvements have plateaued. This shows a need for a renewed effort to address underlying key cardiovascular risk factors, such as endothelial dysfunction, especially since we know that many of them are modifiable and preventable. So then what are the risk factors, and how can we identify and monitor them?
Let’s Talk About Cholesterol & CVD Risk
There are numerous risk factors for heart disease, and to further add to the complexity, some of them, it seems, may not be risk factors at all. Take, for example, the work of Ancel Keys, who’s Diet-Heart Hypothesis led to the implication of dietary cholesterol as a risk factor for CVD. His hypothesis stated that diets high in cholesterol increased blood cholesterol levels, leading to an elevated risk of CVD. This hypothesis has never been proven, and the methodology was ultimately found to be flawed. More recent and better quality research has not shown evidence that dietary cholesterol plays a significant role in the development of CVD. Decades after the original research was published, Ancel Keys himself, in a letter to the the New England Journal of Medicine 1991, stated:
“Dietary cholesterol has an important effect on the cholesterol level in the blood of chickens and rabbits, but many controlled experiments have shown that dietary cholesterol has a limited effect in humans. Adding cholesterol to a cholesterol-free diet raises the blood level in humans, but when added to an unrestricted diet, it has a minimal effect.”
Based on the current weight of evidence, the 2015–2020 Dietary Guidelines for Americans removed the recommendations of restricting dietary cholesterol to 300 mg/day, no longer considering it a nutrient of concern. That’s not to say that blood cholesterol levels are completely inconsequential, but there is more nuance to the subject. It appears the relationship between dietary cholesterol and blood cholesterol isn’t as direct as it might seem. It certainly seems logical that eating cholesterol would raise blood cholesterol levels, but it doesn’t always work that way, except for those with a genetic tendency to be ‘hyper-responders’ (3). In this group, dietary cholesterol does modestly increase both LDL and HDL cholesterol, but it does not appear to affect the ratio of LDL to HDL or increase the risk of heart disease.
In reality, the body tightly regulates the amount of cholesterol in the blood - when your dietary intake goes up, your liver responds by making less. Because of this, foods high in cholesterol tend to have little impact on blood cholesterol levels for most people. This negative feedback loop however can also be mediated by familial hypercholesterolemia (the hyper-responders noted earlier) as well as insulin resistance, metabolic syndrome, which have additional inflammatory components to them.
What we should be Measuring: Particle Size, LP(a) and Oxidised LDL
Far more important than total LDL cholesterol level is the actual particle size. The smaller the particle size, the higher the risk for CVD, so it is these smaller particles that we should be measuring and monitoring. These small particles can get stuck more easily between the endothelial cells of the arteries, contributing to a build-up of arterial plaque. Smaller particles, such as vLDL (very low density lipoprotein) particles, can become oxidised more easily, and these oxidised particles also contribute to the inflammation that can trigger the process of atherosclerosis. Measuring oxidised LDL is therefore an important part of assessing a patients’ risk for cardiovascular events.
Lipoprotein (a) is another particle of concern. It is a type of LDL cholesterol that is considered to be ‘stickier’ than other LDL particles. Testing for Lipoprotein (a) offers a more accurate understanding of CVD risk than testing for total LDL cholesterol alone.
Our Cleveland Heart Lab Advanced Cardio Test is a great comprehensive test for the assessment of cardiovascular risk, covering a range of lipid markers including LDL and HDL particle number and size, Lipoprotein (a), and a number of other metabolic markers including insulin, HbA1c and PLA2.
The Root Mechanism: Endothelial Damage
Rather than trying to create an exhaustive list of CVD risk factors, it is more effective to understand the root-cause mechanisms that underlie potential risk factors.
It is proposed that at the root of all CVD risk factors is a common mechanism, and this mechanism is endothelial damage. The endothelium is a single cell thickness sheath of cells that line the inside of both lymphatic and vascular vessels. The sheath is essentially a barrier that supports transport of materials, immune-related cells such as white blood cells and fluid in and out of the bloodstream. Key aspects of its function revolve around blood clotting, inflammation, vasoconstriction/dilation and angiogenesis. Anything that causes damage to the endothelium may therefore mediate cardiovascular disease risk, and therefore anything that protects the endothelium, such as the nutrients vitamin c and magnesium, has the potential to reduce cardiovascular disease risk. Additionally, factors that increase clotting and reduce endothelial recovery are important to consider when addressing cardiovascular health. It is important to understand that CVD is an inflammatory disease, so when the endothelium is subject to inflammatory processes such as swelling, barrier integrity is affected and the risk of CVD may increase.
Diabetes is one such factor that promotes endothelial damage. Diabetics have a 2- to 4-fold higher risk for cardiovascular events. If the endothelium is exposed to repeated hyperglycaemia, for example, either through Diabetes or the pre-diagnostic stage known as Insulin Resistance, this can promote endothelial dysfunction (2). Additionally, hyperglycemia induces oxidative stress, and may also increase proinflammatory and procoagulant factors and impair nitric oxide release, further adding to CVD risk. Another pro-inflammatory risk factor for CVD is obesity, particularly abdominal obesity, which increases expression of pro-inflammatory cytokines and reduces levels of protective factors including adiponectin. Both Insulin Resistance and Obesity are associated with the condition Metabolic Syndrome.
Genetic factors are also an important area to consider for CVD risk. Methylation - particularly homocysteine - is of vital importance to CVD risk, as high levels promote endothelial dysfunction and coagulation. High levels of homocysteine have been shown to almost double the risk of a cardiovascular event. High homocysteine levels can be a result of one or more genetic variants associated with MTHFR gene that mediates folate processing, or the wider methylation cycle, but is also seen in those with kidney disease, low levels of thyroid hormones and those with dietary folate and B12 deficiencies. Homocysteine is included as a marker on the CHL cardiovascular panels below, and also in our Advanced Coagulation Panel.
Testing Solutions
CVD is a chronic inflammatory disease with multiple lifestyle-related risk factors, including diabetes, dyslipidemia, hypertension and smoking, as well as a host of other risk factors that also promote endothelial damage and increase inflammation. The majority of risk factors for CVD are modifiable, so with the right testing and interventions, outcomes could be greatly improved.
Our most-recommended tests for identifying CVD risk factors are:
- Cleveland Heart Lab (CHL) Metabolic Test
The CHL Metabolic testing includes a CMP and CBC, along with metabolic markers insulin, OxLDL, homocysteine, hsCRP and a full lipid panel. CHL testing also has an advanced cardio option. See a sample report here.
- CHL Advanced Cardio Test
The CHL Advanced Cardio includes a full lipid panel, APOE status, adiponectin, TMAO, Lp(a) and Lp-PLA2 for more advanced cardiovascular assessment. See a sample report here.
- CHL Cardio Elite Profile
The CHL Elite profile includes F2- Isoprostanes/Creatinine, ADMA/SMDA, Lp-PLA Activity2, Ox LDL, hsCRP and myeloperoxidase. See a sample report here.
- Cardiac Puls Test
This test can detect unstable cardiac lesion risk which is the leading cause of heart attack. The PULS test analyses clinically-validated proteins related to inflammation, apoptosis, thrombosis, vascular remodelling. See a sample report here.
We’re happy to help you select the best test for your patient - contact us here to arrange a free support call.
References
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3303762/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2350146/
- https://ebm.bmj.com/content/26/6/295
What's Your Reaction?




















